AstraZeneca said Wednesday night that their coronavirus vaccine was 76% effective against symptomatic COVID-19 cases after U.S. health agencies accused the company of calculating its previously reported rate using “outdated” and “incomplete” data.
The drug maker said two days earlier that its vaccine’s Phase III trials showed it being 79% effective against the virus, but hours later the National Institute of Allergy and Infectious Diseases said that it had possibly relied on “outdated information” in reaching its conclusion. (RELATED: AstraZeneca Cherry-Picked Data, Health Agency Alleges)
In response to the agency’s concerns, AstraZeneca said its data extended through Feb. 17, and vowed to quickly provide more up-to-date information.
In its Wednesday statement, the company said that its vaccine was free of any safety concerns and was 100% effective in preventing severe and fatal COVID-19 cases. It added that it planned to apply for emergency use authorization from the Food and Drug Administration, potentially giving the United States a fourth coronavirus vaccine.
“We look forward to filing our regulatory submission for Emergency Use Authorization in the US and preparing for the rollout of millions of doses across America,” said Mene Pangalos, AstraZeneca’s executive vice president for biopharmaceuticals research and development, in the company’s statement.
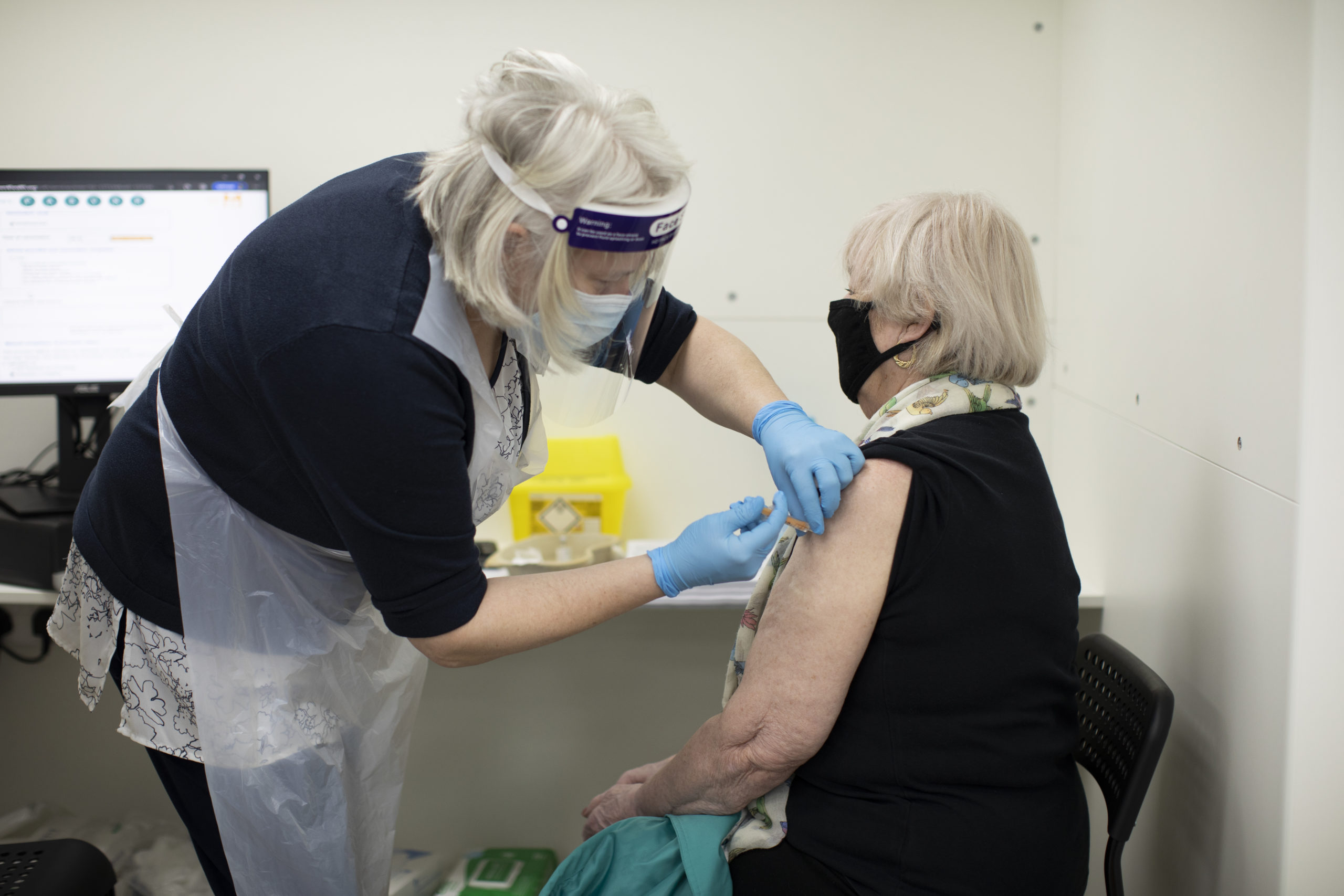
Patients receive the AstraZeneca COVID-19 vaccine at Copes pharmacy in Streatham on February 04, 2021 in London, England. (Dan Kitwood/Getty Images)
Dr. Anthony Fauci, one of President Joe Biden’s top scientific advisors and the director of the NIAID, called AstraZeneca’s misreporting an “unforced error” Tuesday.
“The fact is, this is very likely a very good vaccine,” he said on ABC’s “Good Morning America.” “If you look at it, the data here really are quite good. But when they put it into the press release it wasn’t completely accurate.”
Though AstraZeneca’s vaccine has not yet been approved in the U.S., it has been rolled out in dozens of other countries around the world. In many countries, however, its administration was temporarily paused after reports of blood clots emerged in some people who had received it.
If approved in the U.S., it would join vaccines from Pfizer, Moderna and Johnson & Johnson. Over 130 million doses have been administered nationwide since the first vaccines were approved last December, and approximately 13% of Americans have been fully vaccinated, according to Johns Hopkins University.
All content created by the Daily Caller News Foundation, an independent and nonpartisan newswire service, is available without charge to any legitimate news publisher that can provide a large audience. All republished articles must include our logo, our reporter’s byline and their DCNF affiliation. For any questions about our guidelines or partnering with us, please contact licensing@dailycallernewsfoundation.org.












